When working with complex medical software with a long history, there are bound to be problems. Having
previously embraced an agile approach, the Kappa team’s journey took a disappointing turn over the
previous 5-6 years. The once productive agile team had lost its way. In addition to a massive turn-over
in people, good engineering practices had been replaced by silos and hand-offs. As a result, releases
had become huge, slow, and full of bugs.
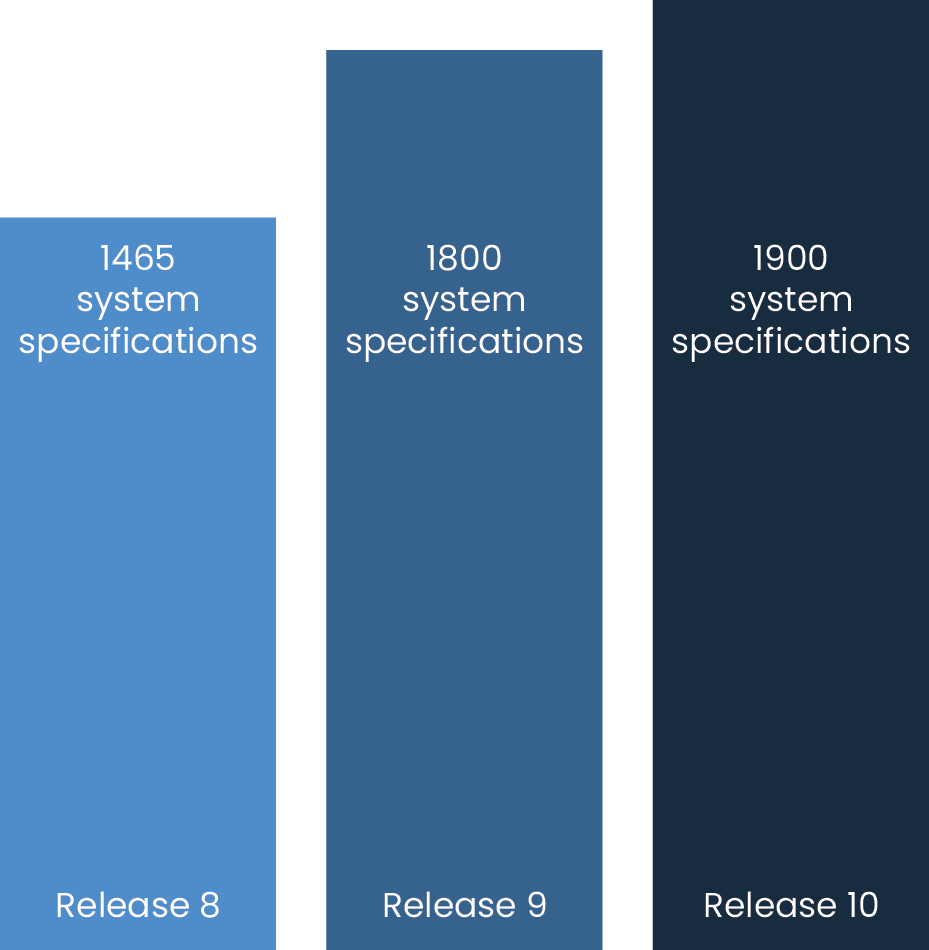
Formal Verification of Release 8 found over 80 major defects. Release 9 had nearly doubled that. Each
defect found would need to be fixed and the software as a whole re-verified before it could be shipped,
creating massive delays. Formal Verification isn’t supposed to be the first time things are tested. It
is
meant to be the
last.
With the releases having become so bug-ridden and having failed an internal audit, senior leadership
turned to Industrial Logic to help the Kappa team improve the quality of their next big software update,
called release 10.